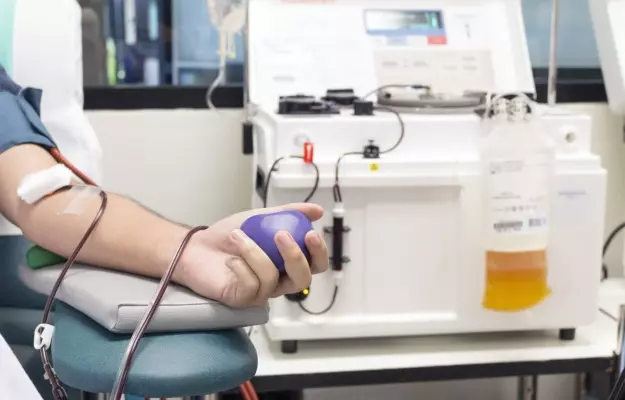
Earlier today (2 July 2020), Delhi chief minister Arvind Kejriwal inaugurated India’s first blood plasma bank for COVID-19 at the Institute of Liver and Biliary Sciences or ILBS hospital.
The move comes in the midst of a global pandemic. The hope is that more and more patients who have recovered from COVID-19 will come forward to donate plasma.
Plasma is the yellowish liquid that’s left over after you remove red blood cells, white blood cells and platelets from blood. In short, it is the thing that makes blood liquid. Plasma contains mostly water, and some blood clotting factors (fibrinogens), electrolytes, proteins like albumin and immunoglobulins (better known as antibodies).
Of late, there has been a lot of interest in blood plasma. The reason: plasma from people who have recovered from COVID-19 may be used in an experimental therapy to treat moderately ill patients who are not responding to any treatment, including steroids like methylprednisolone and dexamethasone.
Here’s how it works: People who recover from COVID-19 do so when their body is able to produce enough antibodies against the infection. These antibodies are transported throughout the body in their blood plasma.
Now, antibodies are usually specific to a pathogen. Let’s stick with the example of viruses. So an antibody for Nipah virus infection would be different from an antibody for Ebola virus disease, swine flu, dengue or COVID-19 and so on.
When you take antibodies from a recently recovered patient and put them in someone else, the antibodies help patient 2 fight off the disease (and the resulting inflammation) for a short time. Ideally, giving patient 2’s body enough time to form its own antibodies and defences against the infection.
This kind of therapy is known as convalescent plasma therapy or passive antibody therapy. It is far from new. In fact, its antecedents go back over 100 years: it was used in the treatment of viral infections like polio, measles and mumps before a vaccine was developed for them.
Doctors became interested in the therapy for COVID-19 in March after medical practitioners in China tried it successfully in the treatment of five critically ill COVID-19 patients who had developed acute respiratory distress syndrome.
In April, India’s apex medical research body Indian Council of Medical Research started clinical trials for COVID-19 convalescent plasma therapy for moderately ill patients.
As it turns out, this was a timely step: since then, the number of COVID-19 cases in India has gone up more than 2400% to cross six lakh at the beginning of July. And the number of people who have recovered from the illness has crossed 3.5 lakh already.
So the question now arises, who among the 3.5 lakh (and counting) can actually donate plasma, for the trial and experimental treatment of other patients who fall sick after them?