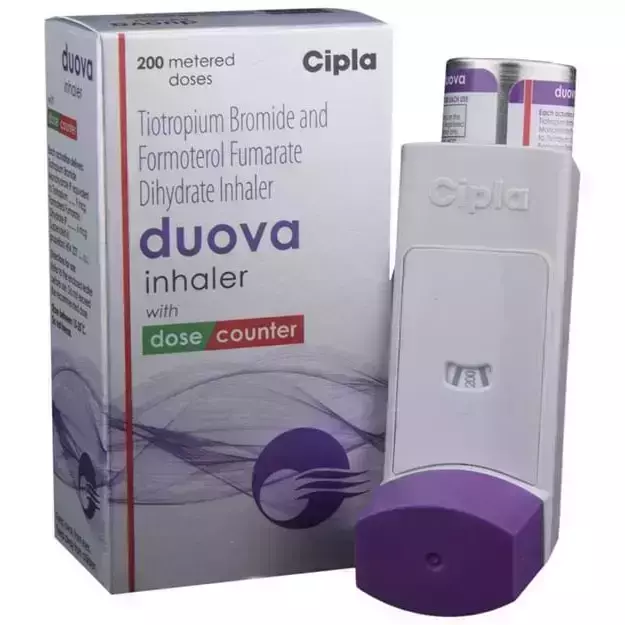
Duova Multihaler (1) is a commercial drug that is prescribed in the form of Inhaler. The alternative uses of Duova Multihaler (1) have also been explained below.
Medical history of the patient along with age and gender determines the dosage of Duova Multihaler (1). Besides the medical condition it is advised for, the route of administration also plays an important role in determining the correct drug dosage. For detailed information on this, read through the dosage section.
While these are the most often observed Duova Multihaler (1) side effects, there are can be others also. These have been listed below. These side effects of Duova Multihaler (1) are usually temporary and subside with the completion of treatment. If, however, they worsen or do not go away, please speak with your physician.
Duova Multihaler (1)'s effect during pregnancy is Severe and Severe while nursing. Warnings related to Duova Multihaler (1)'s effects on the liver, heart and kidney, if any, have been listed below.
Duova Multihaler (1) is not recommended if you suffer from certain medical conditions as it can have adverse effects. Angioedema are examples of such conditions. Other conditions have been mentioned below in the Duova Multihaler (1) contraindications section.
Additionally, Duova Multihaler (1) may also adversely react with other medicines. See below for a complete list.
You should also be aware that Duova Multihaler (1) is safe while driving, and is addiction.
X