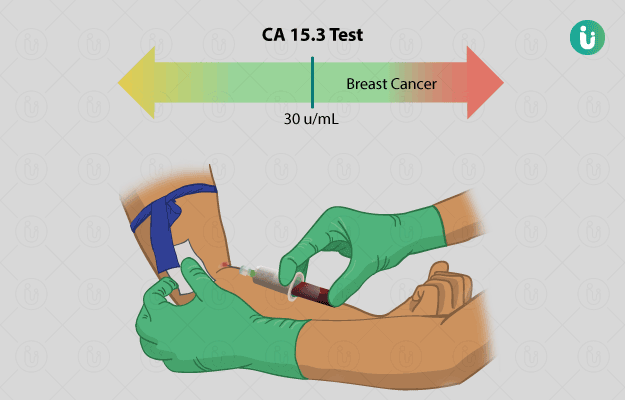
Normal results: CA 15.3 levels below or equal to 30 units per millilitre (U/mL) are considered to be normal and indicate an absence of metastatic (cancer that has spread) or localised (cancer in the particular organ) breast cancer
However, normal levels may also indicate the following:
- A rare case (found in about 20% to 25% of people) in which advanced cancers do not release 15.3 protein
- A very early stage of the disease
Abnormal results: Mild to moderate levels of CA 15.3 indicate the presence of the following conditions:
- Colon, lung, prostate, pancreatic or ovarian cancer
- Hepatitis
- Benign problems in the breast
- Cirrhosis
The highest levels of CA 15.3 indicate that cancer has spread to other organs, such as liver and bones. A constant rise in CA 15.3 levels indicates cancer recurrence or that the treatment provided for cancer is ineffective.
Elevated CA 15.3 levels are observed in about 80% of women with metastatic cancer and fewer than 50% of women with a small tumour in their breast.
A temporary elevation in CA 15.3 levels occurs in some cases of noncancerous diseases, which stabilises over time.
A constant reduction in CA 15.3 levels indicates that the progression of cancer has reduced.
Many factors including noncancerous diseases of liver, breast and ovary, affect test results. So, it is important to check in with a doctor for a correct diagnosis of the condition.
Disclaimer: All results must be clinically correlated with the patient’s complaints to make a complete and accurate diagnosis. This information is purely from an educational perspective and is in no way a substitute for medical advice from a qualified doctor.